- Manufacturing Process
- Why Us
Manufacturing Process
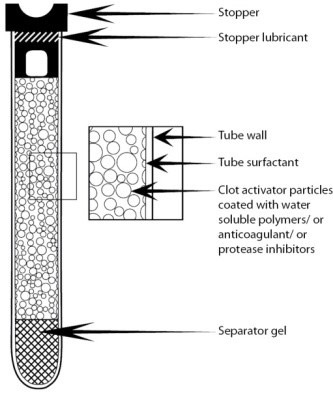
 State of the art ULTRASONIC ATOMIZER NOZZLES used by us produce fine droplets for uniform, controlled coatings on medical and other sensitive and/or small devices. The nozzle spray velocity is low, making it useful for applying thin layers of valuable chemistries with minimal overspray.
State of the art ULTRASONIC ATOMIZER NOZZLES used by us produce fine droplets for uniform, controlled coatings on medical and other sensitive and/or small devices. The nozzle spray velocity is low, making it useful for applying thin layers of valuable chemistries with minimal overspray.
 ULTRASONIC NOZZLES provide excellent repeatability in spray velocity and droplet size for Anti-coagulant (EDTA, Heparin) and Clot-activator (silica) particles. Hence improving sample quality as Anti-coagulant is dispersed through the tubes, abetting adequate mixing interaction between the blood sample and additives resulting in a lower issue of Clots, Micro-clots, cell shrinkage and hemolysis.
ULTRASONIC NOZZLES provide excellent repeatability in spray velocity and droplet size for Anti-coagulant (EDTA, Heparin) and Clot-activator (silica) particles. Hence improving sample quality as Anti-coagulant is dispersed through the tubes, abetting adequate mixing interaction between the blood sample and additives resulting in a lower issue of Clots, Micro-clots, cell shrinkage and hemolysis.
Facility is ISO complain and WHO certified unit.
All the Nidovac™ Blood collection tubes have International traceability with Certification.



A manufacturing facility which has total automation assembly line is well equipped to make Nidovac™ Vacuum Blood Collection a global brand.
Thermo-stable Gel: The Gel material is given at most care so that it behaves at best when used with autoloader and probe aspiration, a special thermo-stable Gel material used in Nidovac™ Gel Vacuum tubes ensure that gel remains in solid state even after centrifugation and produce ‘Zero’ Gel globules.
Why Us
Nidovac™ Vacuum Blood Collection Tubes has many process automation and active ingredients from a Japanese source, resulting in the Japanese quality precision in the Final products.
For better instrument compatibly Nidovac™ Vacuum Blood Collection Tubes have product design, anticoagulant concentration as per the latest CLSI guideline, H1-A5 / GP39-A6 Tubes and Additives for Venous and Capillary Blood Specimen Collection.
This standard contains requirements for the materials, manufacturing, and labeling of venous and capillary blood collection devices. A standard for global application developed through the Clinical and Laboratory Standards Institute consensus process.

Team
Nidovac Vacuum Blood Collection Tubes0 the team has collective experience of more than 30+ years in this industry and many of them have worked with International brands handling Vacuum Blood collection devices in functions like Production, Clinical training and marketing.
Our success is contributed to the fact that we have a better understanding of Preanalytical science, laboratory Accreditations and improving specimen quality. And we are continuing to do solid investment in capability building at our Manufacturing, clinical support team to have better customer experience.


